School of Medicine Columbia
- SC.edu
- Study
- Colleges and Schools
- School of Medicine Columbia
- About
- Faculty and Staff
- Carole Oskeritzian
Faculty and Staff
Carole Oskeritzian, Ph.D.
| Title: | Professor of Pathology, Microbiology & Immunology |
| Department: | Pathology, Microbiology & Immunology School of Medicine Columbia |
| Email: | carole.oskeritzian@uscmed.sc.edu |
| Phone: | 803-216-3462 |
| Fax: | 803-216-3413 |
| Office: |
Pathology, Microbiology & Immunology |

Education
Training:
The Pasteur Institute, Paris, France
Virginia Commonwealth University
Education:
B. S. Biology-Paris-Val-de-Marne University (Paris 12), France
M.S. Biology & Biochemistry- Paris-Val-de-Marne University (Paris 12), France
M.S. Immunology-Denis Diderot University (Paris 7), France
Ph. D. Immunology- Denis Diderot University (Paris 7), France
Research
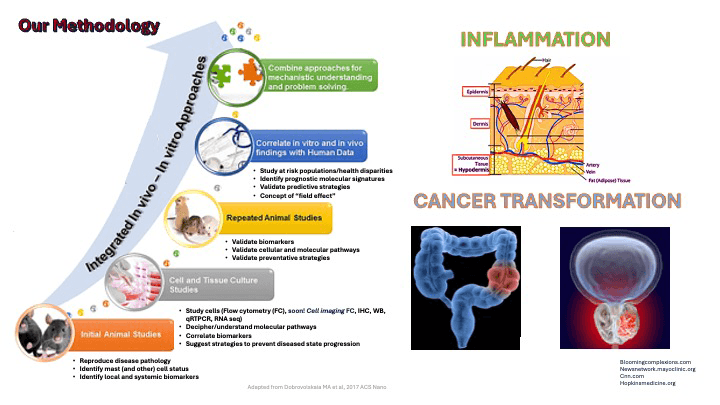
Our team aims to design new preventive strategies for tissue resident innate immune mast cell (MC)-related disorders by identifying local biochemical and molecular pathways underlying the pathogenesis and progression of inflammation and cancer transformation. Our laboratory studies the roles of a sphingolipid metabolite, sphingosine-1-phosphate (S1P) and its receptors, in primary human and mouse MC biology and functions, using genetic, molecular, and pharmacological approaches and human-like disease models. We also aim to understand the molecular mechanisms driving early lung allergic inflammation that we discovered precedes anaphylactic shock as well as the epigenetic regulation of skin inflammation observed in atopic dermatitis or eczema. At USC and beyond, our established expertise in the study of mast cell biology in vivo, ex vivo and in vitro has led to collaborative research with colleagues from institutions throughout the United States. The research conducted in my laboratory has been continually funded by multipleinstitutes at the NIH for over 19 years as principal investigator (PI).
Our current basic science and highly translational projects include measuring the value of MC as prognostic/diagnosticindicators of premalignant dysplasia in colorectal cancer in an IRB-approved human trial conducted in collaboration with clinicians at Prisma Health Midlands. We also investigate the regulatory roles of a natural compound, resveratrol, onskin inflammation, MC activation and S1P production. We are also aiming to develop novel modalities for human prostate cancer screening, using MC as predictors of disease, disease aggressiveness and marks of disease disparity. In collaboration with Dr. Lydia Matesic, Associate Professor of Biological Sciences at the USC McCausland College of Arts and Sciences, we explore the relevance of an innovative approach to regulate MC responsiveness through modulating a molecular signal-controlling hub, the ITCH E3 ubiquitin ligase, predicted to control multiple MC receptor signaling pathways at once, rather than the classical high affinity receptor for IgE, individual mediators or singular signaling pathways involved in mast cell activation and functions. Together, we will identify the underlying mechanisms leading to the pan-inflammatory phenotype featured in human patients carrying deleterious loss-of-function mutations in ITCH.