COVID-19 response: School of Medicine Greenville, College of Engineering step up to coronavirus challenge
Institutions collaborate to improve health outcomes, look to further innovation ahead
Posted on: March 27, 2020; Updated on: March 27, 2020
By Chris Horn, chorn@sc.edu, 803-777-3687
As the coronavirus threatens health and upends daily life, members of the UofSC community family are rising to the challenge with a spirit of resilience and concern for others. See more stories.
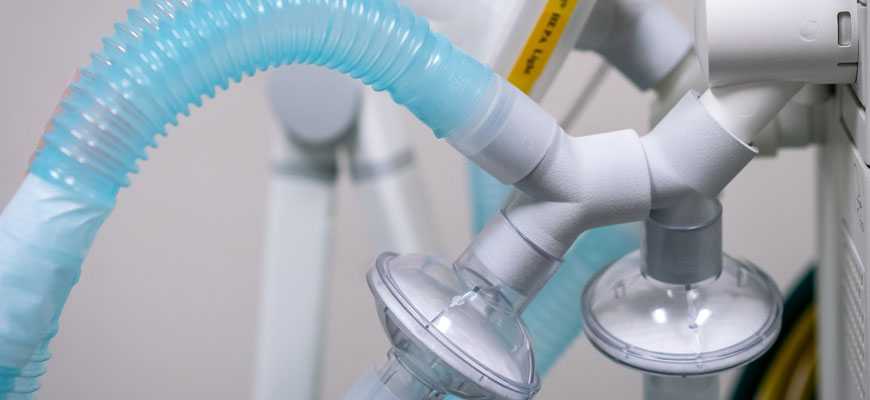
What began as a simple idea from an Upstate emergency medicine physician less than a month ago morphed into a task force of medical and engineering experts focused on one goal — getting emergency FDA approval for a device that enables one ventilator to assist up to four COVID-19 patients.
Prisma Health, the state’s largest hospital system, announced March 25 that it had received federal approval for VESper™, a Y-shaped splitter device that has been approved for use in U.S. locations experiencing a critical shortage of ventilators due to the pandemic. The device looks deceptively simple — almost like a plumbing fixture — but its unique design and the story of how it came to be are a testament to academic teamwork in the face of steep logistical challenges.
“It was two weeks from 3D printing of the prototype to FDA approval,” says Dr. Marjorie Jenkins, dean of UofSC’s School of Medicine Greenville.
It began with Sarah Farris, a clinical assistant professor of emergency medicine at the School of Medicine Greenville, who conceived the idea of a splitter device and shared it with her husband, Ryan, a software engineer. He wrote computer code to 3D print the device and had a prototype made.
Antine Elaine Stenbit, a pulmonology clinical professor at the School of Medicine, was consulted and the device was tested on medical manikins in the school’s simulation center, where Farris also serves as assistant medical director.
“After the team tested the device in the simulation center and determined it performed as expected, Peter Tilkemeier [the School of Medicine’s internal medicine department chair] called me on a Friday night for assistance in connecting with the Food and Drug Administration,” Jenkins says. “I worked with the Office of Women’s Health in the Office of the FDA Commissioner until moving to Greenville last July. By 10 p.m. that same night, I was on the phone with FDA Department of Devices and Radialogical Health director Jeff Shuren.”
By 2 p.m. the next day, Jenkins and her small crew were teleconferencing with FDA officials, then convened a wider group by the next day. What followed was a fast-moving yet focused series of consultations on everything from 3D printing logistics with UofSC mechanical engineering faculty to forward-looking considerations such as design improvement when the first field data begins to emerge.
““We have been evaluating different 3D printing technologies and coatings of printed parts to ensure they are safe for medical use,” says Wout De Backer, a research assistant professor in the College of Engineering and Computing’s mechanical engineering department. “The high porosity of many 3D printing technologies allows bacteria to nestle in the voids, so selecting the right combination of manufacturing technologies is critical.”
The design team has incorporated HEPA filters on Prisma Health’s VESper™ device, Jenkins says, which capture bacteria and virus particles and helps prevent cross-contamination with other patients connected to the same ventilator.
Neset Hikmet, a professor in the College of Engineering and Computing and director of Applied Sciences at the Center for Innovation and Advanced Analytics, says the device could potentially extend hope far beyond the United States.
“When you look at less fortunate countries that can’t afford to purchase new ventilator equipment, this approach lets them quadruple their ventilator capacity,” Hikmet says. “What we’re doing to innovate and improve has a global impact — that’s the exciting part.”
For now, Prisma Health experts are working with national COVID-19 teams during field-testing to monitor clinical outcomes. Those field tests will determine whether the device performs as designed, per FDA guidelines. University consultants such as Hikmet will use that data to suggest potential improvements to the device in the weeks ahead.
More than 30 requests had been made — either for the 3D printing code or the device itself — in the first day after the VESper™ device was announced, Jenkins says. The Sargent Foundation of Greenville has already made a six-figure donation to the School of Medicine Greenville to cover the costs of producing the device for hospitals that don’t have access to 3D printing capabilities. A statement from the foundation read: "We are proud to support such an innovative and timely endeavor that will benefit our community and nation in this time of crisis.”
The School of Medicine Greenville is also looking into other innovations related to the COVID-19 pandemic, including rapid turnaround test kits.
“We’ve formed a rapid innovation group here that meets every day to address ideas from the field that people are telling us they need as they rapidly mobilize,” Jenkins says. “Students from our campus and the medical campus in Columbia are volunteering in the hundreds to help the effort. I think the University of South Carolina has shown up in an amazing way.
“I’ve been on the phone with President (Bob) Caslen a number of times, and he said to do whatever you need to get this done. He is just phenomenal.”