Hematopoiesis is the process by which hematopoietic stem cells (HSCs) give rise to all mature blood cells. HSCs simultaneously maintain quiescence and promote activation through tightly regulated cell-extrinsic and cell-intrinsic frameworks of proteins that maintain, promote, and repress specific gene expression patterns to enable production of blood for the lifespan of an organism. This delicate balance allows for maintenance of a constant source for immune cell production while retaining a robust stem cell pool. Disruption of the signaling pathways that maintain the stem cell pool can have significant consequences on the responsiveness, longevity, and strength of the immune system, often resulting in HSC exhaustion through chronic activation. Defining the mechanisms that regulate stem cell maintenance, proliferation, and differentiation is critical for identifying therapies for improving stem cell function under stress.
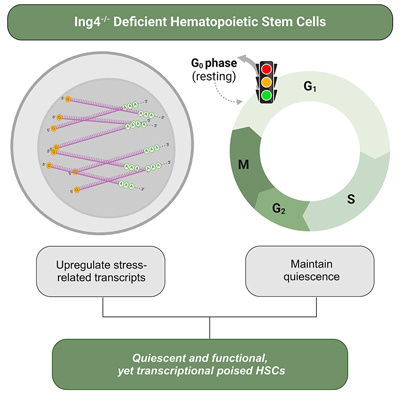
In their new study titled "Ing4-deficiency promotes a quiescent yet transcriptionally poised state in hematopoietic stem cells" and led by former graduate student Dr. Zanshé Thompson and current graduate student Georgina Anderson, the Kathrein lab identified the tumor suppressor, Inhibitor of Growth 4 (Ing4), as a critical regulator of hematopoietic stem cell (HSC) homeostasis. Ing4 deficiency in HSCs promotes gene expression signatures associated with activation, yet HSCs are arrested in their cell cycle and express several markers of quiescence. Functionally, Ing4-deficient HSCs demonstrate a robust regenerative capacity following transplantation. Overall, results from this study suggest that Ing4 deficiency promotes a poised state in HSCs, whereby HSCs appear transcriptionally primed for activation, but remain in a resting state.