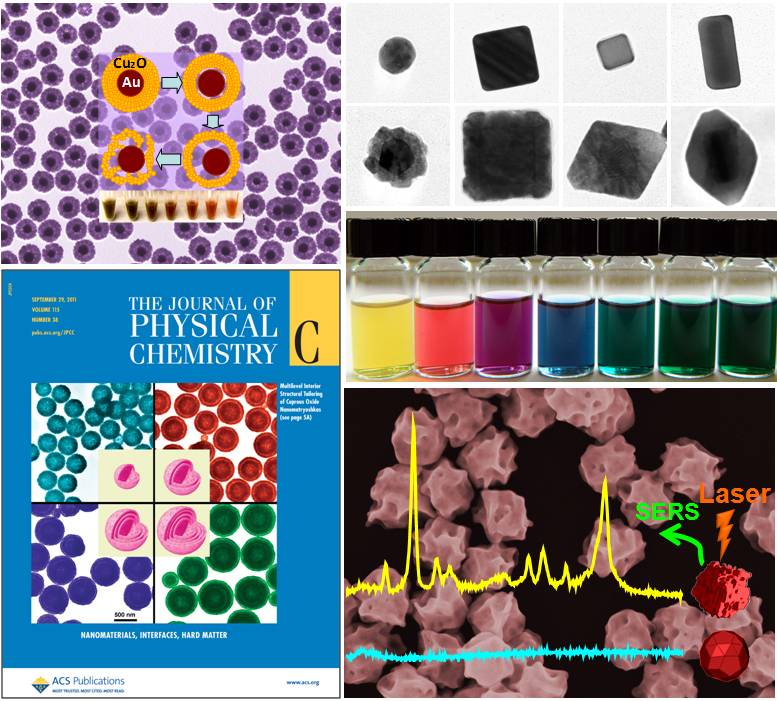
Nanophotonic materials have emerged as an important class of subwavelength optical components that interact with light in unique ways on the nanometer length-scale. Our interests in nanophotonic materials broadly span several important aspects in this field, including but not limited to nanostructure fabrication, nanoscale self-assembly, structure-property relationship, and applications of these materials. We have been working on several nanostructures with geometrically tunable optical properties, such as semiconductor nanoshells, metal-semiconductor core-shell heteronanostructures, and complex metallic nanoparticles. We are particularly interested in (i) development of new approaches for the controllable fabrication of optically active nanostructures; (ii) utilization of optically active nanostructures as building blocks to assemble mesoscopic structures over multiple length scales; (iii) detailed characterization of the optical properties of these nanomaterials both at the ensemble and single-nanoparticle levels; (iv) development of quantitative understanding on the structure-property relationship of these nanostructures through combined experimental and theoretical efforts; and (v) applications of these nanophotonic materials in surface-enhanced spectroscopies for ultrasensitive molecular sensing.
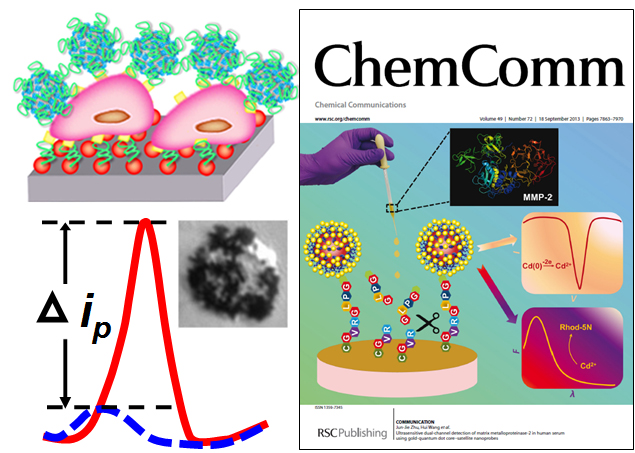
The past two decades have witnessed significant progress on the development of robust analytical tools with high sensitivity, selectivity, and reproducibility toward early diagnosis of cancer. Electrochemical cytosensing has emerged as an extremely attractive method that can be readily implemented into quantitative bioassays for high-throughput clinical applications. Utilization of rationally designed multifunctional nanoprobes and specifically tailored nano-biointerfaces for electrochemical cytosensing provides unique opportunities to optimize the interfacial electron transfer and cell recognition processes, allowing for the integration of large signal amplification, enhanced detection specificity, and expanded multiplex sensing capabilities on one cytosensor. Our group has been working on the design and fabrication of multifunctional hybrid nanoprobes for selective and ultrasensitive electrochemical detection of a variety of cancer cells, such as leukemia cells and circulating tumor cells. Our electrochemical approaches also allow for the quantification of the expression levels of important biomarkers on the cancer cell surfaces.
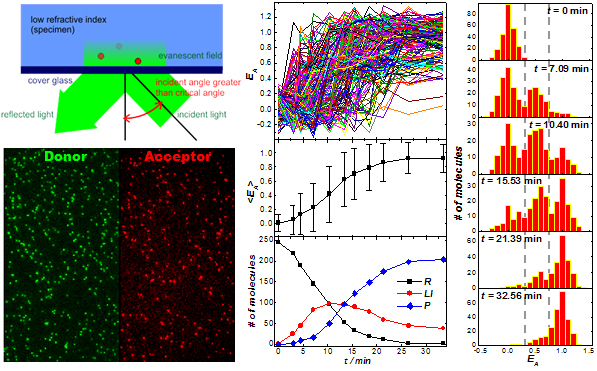
Our group has been working on the development of detailed, molecular-level understanding of important nucleic acid (NA) structural remodeling processes chaperoned by retroviral nucleocapsid (NC) proteins. Rretroviral NC proteins promote several NA structural remodeling processes that are crucial to the retroviral life-cycles, such as the obligatory strand transfers in the reverse transcription, the maintenance and integration of proviral DNA, and the genomic RNA protection and packaging. NC-chaperoned NA structural remodeling typically involves various intermediates along multiple reaction pathways and is tightly associated with heterogeneous conformational dynamics over multiple time-scales that cannot be synchronized and resolved by ensemble measurements. Single-molecule spectroscopy provides an extremely powerful way to characterize such complex biomolecular processes without the ensemble averaging effects. Our group uses time-resolved single-molecule spectroscopy as an analytical tool to study the kinetics and mechanism of NC-chaperoned NA annealing that occurs during the reverse transcription and to resolve the conformational dynamics associated with NC-induced local bending of proviral DNA. These single-molecule measurements allow us to gain molecular-level insights on the complicated, dynamic NC-NA interactions that underpin NCs’ NA chaperone functions.