
Mrinmay Chakrabarti, PhD
Research Assistant Professor
Our vision is to improve the understanding and treatment of inflammatory vascular diseases by studying the vascular response to thrombosis.

Dr. Colin Evans is a lead faculty member of the Department of Cell Biology and Anatomy and the Cardiovascular Translational Research Center in the School of Medicine at Columbia. He is also an affiliate faculty member of the Department of Biomedical Engineering in the College of Engineering and Computing.
During his postdoctoral training, Dr. Evans received research funding from the British Heart Foundation, the British Society for Haematology, the University of Cambridge School of Biological Sciences, and the American Heart Association. He was then recruited to the University of South Carolina as a Principal Investigator.
Dr. Evans serves on the Editorial Boards of Thrombosis Journal, Thrombosis Research, and Thrombosis Update. He is an Associate Editor of Frontiers in Cardiovascular Medicine and serves on the Early Career Editorial Board of Arteriosclerosis, Thrombosis, and Vascular Biology. He has obtained grant and travel support from more than 15 sources and has published more than 60 papers in peer-reviewed journals.
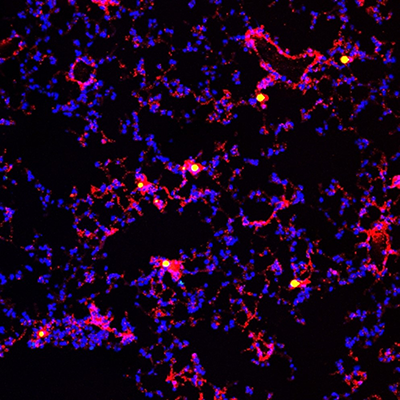
Pictured is an inflammatory lung that has suffered a sepsis challenge. The lung contains nucleated cells (blue) and endothelium-lined blood vessels (red). Some of the lung blood vessels are occluded by thrombotic polymer microbeads (yellow).
How do blood vessels respond when blocked or injured? Can this response be leveraged to treat inflammatory vascular diseases? The Evans Laboratory studies the vascular response to thrombosis, with a view to improving treatments for inflammatory vascular diseases. We focus on two discrete but related disease areas: (i) inflammatory lung injury and repair; and (ii) thrombus formation and resolution.
Inflammatory Lung Injury and Repair
Inflammatory lung injury includes acute lung injury and acute respiratory distress syndrome (ARDS). There are currently no effective treatments for ARDS, which has a mortality rate of approximately 40%. Cells that line the inside of blood vessels are called endothelial cells. In acute lung injury and ARDS, some endothelial cells in the lung fail to survive. For the injured blood vessels in the lung to subsequently repair, the lung endothelial cells must regenerate. If endothelial cell division in the lung is insufficient, however, these blood vessels will not successfully repair.
The Evans Laboratory uses clinically relevant models of inflammatory lung disease, and integrated biochemical and molecular analyses, to study how endothelial cell survival and regeneration is regulated in the inflammatory lung. We also use targeted gene editing and drug delivery techniques to improve endothelial cell survival and regeneration as potential therapeutic strategies for inflammatory lung injury. These studies could lead to the development of novel treatments for acute lung injury and ARDS.
Thrombus Formation and Resolution
Venous thromboembolism includes venous thrombosis and pulmonary embolism. There are currently no safe and effective treatments for deep vein thrombosis (DVT), which is a painful and debilitating condition that can lead to pulmonary embolism and post-thrombotic syndrome. When endothelial cells lining the inner wall of blood vessels are disturbed, they trigger coagulation and thrombus formation. For blood flow to be restored after a DVT, the thrombus must be removed or remodel and resolve. Venous thrombus resolution involves contraction of the thrombus away from the vein wall and the formation of endothelial cell-lined channels within and around the thrombus. If thrombus resolution fails, however, venous valve damage and chronic thromboembolic pulmonary hypertension can occur.
The Evans Laboratory uses clinically relevant models of thrombosis, and complementary molecular and biochemical analyses, to study how venous thrombus formation, vascularization, and resolution is controlled. We also use targeted gene therapy and drug delivery approaches to inhibit thrombus formation or improve thrombus vascularization as putative therapeutic strategies for venous thrombosis. These studies could lead to the development of a novel treatment for DVT.
Research in The Evans Laboratory is currently funded by:
American Heart Association
National Institute of General Medical Sciences
University of South Carolina, School of Medicine
University of South Carolina, Office of Undergraduate Research
University of South Carolina, Office of the Vice President for Research
University of South Carolina, Honors College
View Dr. Evans’ full list of publications on his MyBibliography page in NCBI.
Selected awards and news items are highlighted on Dr. Evans’ Faculty Profile and X / Twitter account.
Latest News, Honors, and Coverage
February 2026: Dr. Evans is selected as a Finalist for the 2026 Kenneth M. Brinkhous Early Career Investigator Prize in Thrombosis at AHA Vascular Discovery.
February 2026: Isabella Avosso (Undergraduate Research Assistant) is accepted onto the DAAD RISE Germany Program.
January 2026: Dr. Evans is interviewed on Discover CircRes, a podcast of the American Heart Association Journal, Circulation Research.
September 2025: Dr. Evans is elected a Fellow of the American Heart Association (FAHA).
August 2025: Dr. Evans is selected as the Institutional Candidate for the Pew Scholar and Mathers Foundation Awards.
December 2024: Seth Gould and Ansley Herron (Undergraduate Research Assistants) are awarded a Magellan Scholar Award.
July 2024: Dr. Evans receives a Transformational Project Award from the American Heart Association.
July 2024: Dr. Evans receives an Advanced Support for Innovative Research Excellence (ASPIRE) Award from the University of South Carolina.
July 2024: Dr. Evans joins the ATVB Early Career Committee of the Council on ATVB of the American Heart Association.
July 2024: Dr. Evans and his research are highlighted by the Critical Care Assembly Early Careers Professional Working Group of the American Thoracic Society.
May 2024: Shriya Gandham and Seth Gould (Undergraduate Research Assistants) are awarded South Carolina Honors College Research Grants.
January 2024: Dr. Evans is selected to serve on the Early Career Editorial Board of Arteriosclerosis, Thrombosis and Vascular Biology, which is a peer-reviewed journal of the American Heart Association.

Research Assistant Professor

Postdoctoral Fellow

PhD Student

PhD Student

Undergraduate Research Assistant

Undergraduate Research Assistant

Undergraduate Research Assistant

Undergraduate Research Assistant

Undergraduate Research Assistant